When humans begin to “create” new animals, with genetic engineering, it is a relatively fast result taking a generation or two of animals. But the reality is that humans have been engineering animals since about 11,000 years ago when the first domesticated goat was developed, considered to be the first domesticated animal for supplying food to humans.1 Selective breeding is also a technology that is used to deliberately create a version of the animal that you “select for”. However, when genetic engineering reaches across species line to other animals or even insects with which an animal could never breed with in reality, that begins to raise eyebrows.
But what if you could make an animal that would produce a useful drug to treat disease in humans? How different is that from producing an animal solely to be killed for food for humans? In the first example, the animal continues to live!
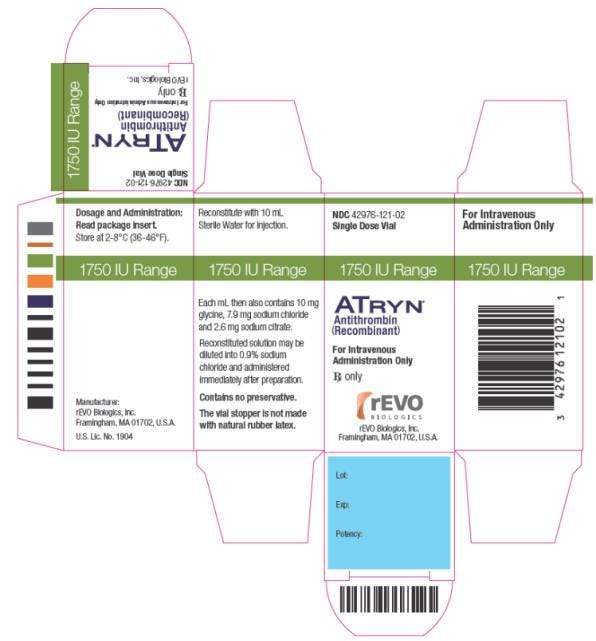
In 2009, the FDA issued its first approval of a biological product derived from genetically engineered animals.2 ATryn, produced by GTC Biotherapeutics, Inc., is an anticoagulant used for the prevention of blood clots in patients with hereditary antithrombin (AT) deficiency, a rare blood disorder affecting approximately 1 in 5,000 people in the United States. ATryn is produced from the milk of transgenic goats that have been genetically modified to contain DNA for the production of human antithrombin. The manufacturer of ATryn received approval from two FDA centers that separately evaluated the human orphan drug and animal drug aspects of the biologic. The Center for Biologics Evaluation and Research (CBER) evaluated the biologic’s safety and efficacy in human clinical studies, and the Center for Veterinary Medicine (CVM) addressed the safety and stability of the recombinant DNA construct in the transgenic goats.
The CBER evaluated two studies that included 31 patients with hereditary AT deficiency, with only one ATryn-treated patient developing the blood clots that ATryn is designed to prevent. During its review, the CVM determined that introduction of the construct did not cause any adverse outcomes to the health of the transgenic goats and ensured that the manufacturer had adequate procedures in place to prevent food from the goats from entering the food supply.
Furthermore, the CVM specified that the goats could not be used for food or feed and validated a method suitable for identifying the DNA construct in both animals and their products. The CVM also conducted an environmental assessment under NEPA, finding that the goats did not cause any significant impact on the environment.
The issues of the welfare of the animal, as well as the risk to the environment were considered in this Environmental Assessment. Several steps to ensure safety included segregation of the animals. Here is an excerpt from the Environmental Assessment, January 2009:
D. Phenotypic Characterization of the GE Animal
CVM reviewed all of the information submitted by GTC to the agency in order to characterize the phenotype of the GE animals to determine whether the insertion of the Bc6 rDNA construct or its expression may have caused changes resulting in the increased risk of adverse outcomes. This review included the results of both of the FDA inspection and the site visit of the GTC farm in Massachusetts. Particular emphasis has been placed on the visit conducted by a CVM veterinarian and a ruminant animal physiologist in November 2008, in order to observe goat management procedures, review original animal health and husbandry records, and obtain copies of standard operating procedures and internal GTC reports related to the health and husbandry of the GTC 155-92 GE goats.
CVM’s review indicated that there were no apparent differences in the health, mastitis, nutrition, and reproductive status of GTC 155-92 goats vs. non-GE goats on GTC’s goat farm. Other than the presence of rhAT in the milk of the GE goats, which is the intended outcome, the only difference noted was that rhAT female goats had lower daily milk production and shorter lactations than their non-GE herd mates. This is attributable to genetics that originated from a single male founder vs. their non-GE herd mates that had a more diverse genetic background with increased opportunity to introduce superior dairy genetics vs. the rhAT population.
CVM concluded that the insertion of the Bc6 rDNA construct did not pose an increased risk of adverse outcomes to the health of the GE animals; no effects were noted that are anticipated to have an adverse outcome on the environment.
Atyrn was approved by the European Commission in 2006 and by the U.S. FDA in 2009.3 Here is the approved labeling and packaging, appearing like any other drug:
The Goat as a Gift
Indigenous peoples almost consistently regard nature and the Creator as giving gifts of life to humans through animals, plants, nature, climate and all of the ecology, considered relatives. Reciprocity is an important part of giving something back for that gift, whether it is a gift of sacred tobacco as a way of giving thanks, or it is grooming and feeding the goat, for example. Was it not enough of a gift that the goat gives milk and meat to sustain humans? Is it ungrateful of humans to alter this gift to change the milk into something that cannot be consumed, after it has been altered to contain a drug? If you have a family member suffering from this rare and potentially deadly disease, then having more affordable and available anti-thrombin drug is a blessing.
In 2009, the production of ATryn started with a herd of 200 goats in central Massachusetts. They also have herds in the UK and France. GTC and its successor has continued to work on new uses and products, including an undisclosed new use for ATryn. It also licensed a compound called ublituximab to TG Therapeutics Inc. as a possible treatment for cancer and autoimmune diseases.
GTC has a 11,500-square-foot facility in its Charlton farm, for farming 1,000 to 1,200 rabbits for its Factor VIIa program, having completed phase 1 of the drug approval process with U.S. FDA. At the time of the approval, the rabbits making the milk were in a colony in France.4
In 2013, GTC Biotherapeutics, an LFB Group Company, announced a Name Change to rEVO Biologics and Renewed Commitment to Evolving Recombinant Medicine.5 In 2014, the US Food and Drug Administration (FDA) approved the second milk-derived drug from a transgenic animal (rabbit), conestat alfa, for the treatment of hereditary angioedema.6
BTC Therapeutics, the manufacturer of ATryn has had losses every year from the drug, and expects to have those years of losses, but it has been enough to discourage a rush to this method of drug production.
Evidence suggests that from two million years ago to 12,000 years ago, humans were hunters and gatherers. Around 10,000 years ago, humans began both the selective crossing of plants for crops and the domestication of animals through selective breeding. Sheep and goats were domesticated around 9,000 B.C.; swine around 7,000 B.C. and cattle around 6,500 B.C. Hawkes K, O'Connell J, Blurton Jones N. Hunter-gatherer studies and human evolution: A very selective review. Am J Phys Anthropol. 2018;165(4):777-800. doi:10.1002/ajpa.23403.
https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/atryn
https://www.reuters.com/article/lifestyle/science/us-approves-first-drug-from-dna-altered-animals-idUSTRE5154OE/
https://www.telegram.com/story/news/local/north/2012/03/25/new-life-found-at-biotech/49693040007/
https://www.biospace.com/gtc-biotherapeutics-an-b-lfb-group-company-b-announces-name-change-to-b-revo-biologics-b-and-renewed-commitment-to-evolving-recombinant-medici
https://www.nature.com/articles/nrd4426